ClearMasks trial
The Department of Health & Social Care has purchased half a million ClearMasks[1] however, only about 50,000 have been delivered so far. Supplies of these are being manufactured rapidly but for the time being their use should be targeted in your organisation to where they will support patients, service users and staff.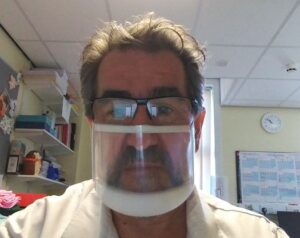
Approval for use of ClearMask face masks
ClearMask face masks are approved by the Health and Safety Executive (HSE) for use during the COVID-19 pandemic under the essential technical specification guidance.
Upon reviewing the ClearMask product documentation and assessing the intended use of ClearMask against the essential health and safety requirements of the EU PPE regulation, the HSE has approved the availability of ClearMask face masks via NHS Supply Chain for use in healthcare settings as an item of PPE to protect the wearer.
Please note: ClearMask face masks are not currently CE marked which is why HSE approval was sought.
Where can I use a ClearMask?
The ClearMask has a clear front, making the mouth and face visible to facilitate communication with service users who rely on lip-reading and use facial expression to support communication; this includes people who are d/Deaf, have a learning disability, autism and dementia.
The ClearMask face mask provides splash protection for the wearer in the same way that a type IIR surgical face mask does: (COVID-19: infection prevention and control (IPC)) However, since the product has not been tested by regulators in the same way as a type IIR or type II surgical face mask, to show protection from the wearer to the patient,[2] health and social care providers must risk assess the appropriate use of the product.
They should not be used:
· in the surgical/operating setting or for surgical/invasive procedures;
· where there is excessive splashing or spraying of body fluids (as potential splash-back from the impervious front);
· as an alternative to a filtering face-piece respirator, which is worn to protect the worker during aerosol generating procedures.
The manufacturers state that ClearMask is a ‘single use’ product and should not be reused, however some trusts are classing them as sessional.
ClearMasks are not fitted (donned) in the same way and if they are not fitted properly, there may be a risk of slipping from the nose or restricting breathing. Instructions are available on the ClearMask website here or a training presentation has been created by Sarah Bent from Betsi Cadwaladr. Watch the video
There is also now an official national survey link for feedback directly to DHSC: Provide your feedback here
Feedback has been requested by 23rd Sept, however, DHSC have said: “We are asking for completions by Wednesday 23 September but understand that these are short timescales as we haven’t yet concluded all of the pilot drops, so if providers need a bit more time to complete then that’s no problem.”
There is a discussion on our the BAA Facebook group about the use of the ClearMasks in departments if you wish to see what UK audiologists are saying about the product.
A spokesperson from HSE has said: “I can confirm that at the request of DHSC, HSE have applied a regulatory easement to allow this non-CE marked product (ClearMask) into the healthcare supply chain as a piece of PPE. This easement is only applicable for the duration of the COVID-19 pandemic and then the product can no longer be used (unless it obtains CE marking in the interim).
“It provides splash protection for the wearer that is equivalent to a Type IIR surgical mask. It has not been assessed against EN14683 for use as a surgical mask to protect others from the wearer.
When we applied the easement to this product, we considered that based on the PPE Ensemble Guidance, the product could be used on COVID wards (in place of a Type IIR mask) , but not where AGPs were being carried out. Since then though, SoS guidance has changed on the use of face coverings in hospitals. If you intend to use this product as a face covering in non-clinical areas, then it is not being used as PPE, and there is no requirement for products to be CE-marked.”
[1] Distributed by ClearMask, LLC, 900 E. Fayette St., PO Box 22493, Baltimore, MD 21203, USA [2] The PPE Ensemble Guidance details where Type II and Fluid Resistant Type IIR surgical masks are typically used. ClearMask face masks have not been assessed for bacterial filtration efficiency, to determine source protection from the wearer to the patient, as a surgical mask. ClearMask does however protect the wearer from splashes in the same way that a Fluid Resistant Type IIR surgical mask does.
Today is my 1st day with them, I have tried to wear one all day (as is now our trusts policy) and have not noticed any problems with fogging! We have Perspex screens on the desks in our department and using the "normal" mask lead to lots of problems. There has been noticeably less "pardons" today so, so far so good!
Adrian Lodge, Senior Audiologist at United Lincolnshire Hospitals NHS Trust (pictured above wearing a ClearMask)
