Outline of Process for Seeking NHS Research Ethics Approval
This brief simple guide is to outline the various stages involved in the of process to apply for NHS research ethics approval which may be local studies or more complex multi-centre studies. Detailed information for all stages of research studies can be found on the Health Research Authority website: www.hra.nhs.uk
Stage 1: Determine if proposed study is classed as “research” by the Health Research Authority (HRA).
The managing organisation is responsible for determining whether a project is classed as research by the HRA and if so, the managing organisation would accept the role of sponsor. The HRA provides an online decision tool and a specific one for student projects, to help determine whether or not study is research as defined by the UK Policy Framework for Health and Social Care Research. It is based on the Defining Research table produced by the Research Ethics Service and will provide a “YES” or “NO” answer.
If “NO” NHS REC review is not required for study.
If answer is “YES” next question is “Do I need NHS REC review”? which is also determined by the provided online decision tool, which is based on whether the study is classed as research and involves one or more of the following:
- A clinical trial of an investigational medicinal product (CTIMP).
- A non-CE marked medical device; or a CE marked device, which has been modified or is being used, outside of its current intended purpose.
- Study involves exposure to ionising radiation.
- Study involves the processing of disclosable protected information on the Register of the Human Fertilisation and Embryology Authority (HFEA) by researchers, without consent?
This decision tool only determines if study requires NHS REC review, however, need to check if other approvals may be needed.
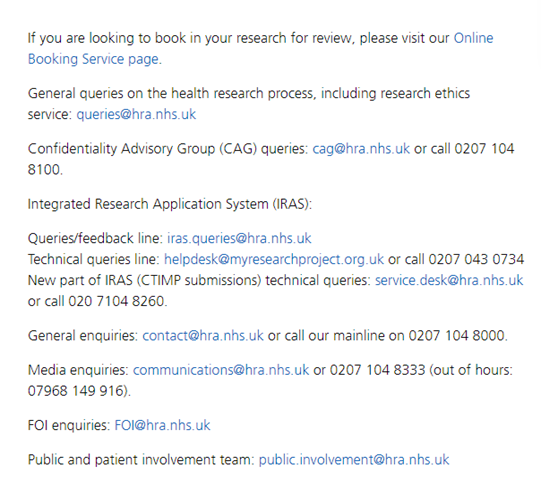
Stage 2
For studies requiring NHS REC review:
- For first time users set up an IRAS online account and “Login” details
- Prepare all documentation required ready to be uploaded with online submission. Documentation can vary depending on study but generally includes study protocol, study handbook, investigator CV, patient information sheet and consent form, study costs, any contracts and study agreements.
Stage 3
For studies requiring NHS REC review complete form online using:
- The standard Integrated Research Application System (IRAS).
OR
- Apply for “Combined Review (REC+MRHA)” for Clinical Trials of Investigational Medicinal Products (CTIMPs) and Combined trials of an investigational medicinal product and an investigational medical device (IMP/Device trials)
- Apply for “Combined Review” for studies using Ionising Radiation.
- Apply for fast tracked “Combined Review” for Covid-19 research.
Stage 4
When IRAS application completed and all required documentation has been uploaded, submit application with request option for:
- Proportionate Review process for studies where the proposed research raises only minimal ethical risk, focusing on minimally sensitive topics; entail minimal intrusion or disruption to others; and involve participants who would not be considered vulnerable in the context of the research.
Or
- REC ethical review
Stage 5
- Applications are reviewed by REC and/or MHRA committees (which are usually held virtually) and its advisable for investigator to attend when invited, (not applicable to Proportionate Review applications).
- The REC Committee must notify of their decision within 60 calendar days of receiving your valid application and 21 days for studies accepted for proportionate review. After meeting, you will be notified of the REC’s decision, usually within 10 working days, however MHRA decisions may take longer.
- If REC/MHRA further information or study amendments required details of timelines for receipt of corrections will be provided.
Contact details from HRA