Scope of Practice update
BAA Process for developing the revised Scope of Practice (ScOP) Document
It is recognised that the Scope of Practice document is a critical piece of work in defining the boundaries of knowledge, skills and responsibilities for audiologists practising in the UK.
Given the complexity and importance of this document, we are proposing a more structured development and consultation process to ensure that the new Scope of Practice document effectively meets the needs of audiology services and services users.
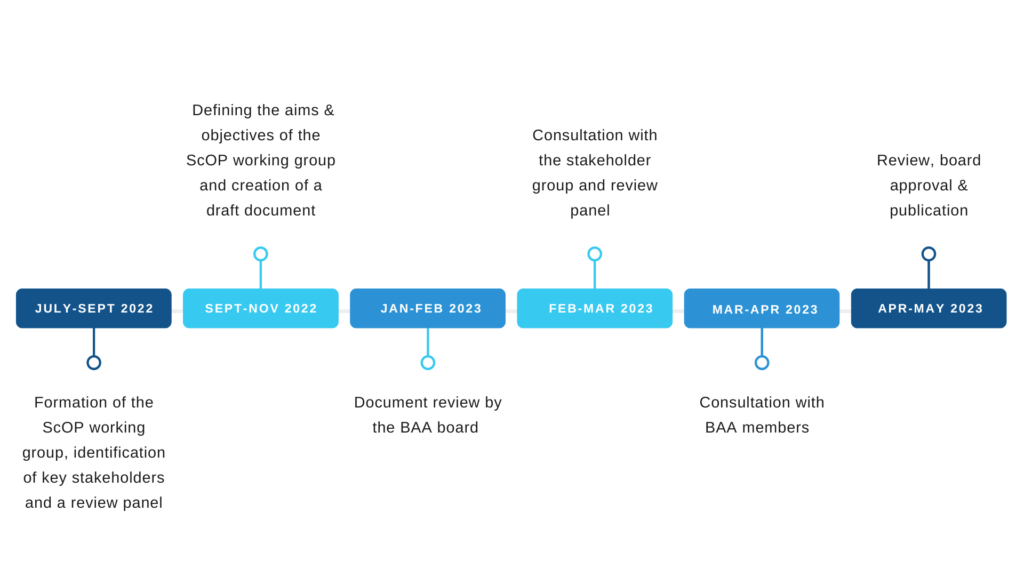
Figure 1 The proposed timeline of the document development process
Stage 1: Formation of the ScOP working group, identification of key stakeholders and a review panel
July-September 2022
The working group should be formed with representation from across sectors, specialities and the four nations. An additional stakeholder group is required for feedback on drafts (e.g., Health and Care Professions Council (HCPC), Registration Council for Clinical Physiologists (RCCP), Academy for Healthcare Science, British Society of Audiology (BSA) and British Academy of Audiology (BSHAA)) and a review panel to ensure the document is clear, jargon-free and sufficiently detailed.
Stage 2: Defining the aims & objectives of the ScOP working group and creating a draft document
September-November 2022
The working group will first agree the role of the chief editor and each team member.
The group will be required to define what an “audiologist” is for the purpose of this document, and establish the professional contexts that will be considered within this scope of practice. Previous versions of the document will be reviewed alongside the standards of proficiency from Professional and Statutory Regulatory Bodies (PSRBs).
The ScOP working group should review the draft document until either a consensus is reached, or key issues are identified for consideration by the board or advisory/stakeholder groups. Until approval, all documents should be clearly marked as draft using a watermark.
Stage 3: Document review by the BAA board
January–February 2023
Once the working group has agreed that a draft is ready for review, the document will be circulated among the BAA board only for initial comments. Once these comments have been addressed by the ScOP working group and approved by the board, the draft can be considered by stakeholders and the review panel.
Stage 4: Consultation with the stakeholder group and review panel
February-March 2023
As for stage 3, the stakeholder group and review panel will be asked to provide feedback on the draft. BAA board director of the responsible for the document will invite the organisation or individual to provide an opinion on the document content and/or suggestions for improvement. This may be on the whole document or on specific areas of the document. The lead author will clearly indicate this when inviting comments. The invitation to comment will usually be via an email, and a deadline by which comments should be submitted to the BAA email address will be clearly indicated. Once comments have been satisfactorily addressed by the ScOP working group, the draft document will be sent out for wider consultation.
Stage 5: Consultation with BAA members
March-April 2023
A draft version of the document will be available on the news pages of the BAA website (www.baaudiology.org) and information about the consultation will be provided through Horizons and the BAA social media channels. The timescale for consultation will be four calendar weeks.
Stage 6: Review, board approval and publication
April-May 2023
Following consultation, a revised document from the ScOP working group will be sent to the board for final comments and approval. Once the board approves, the document will be published alongside supplementary materials and webinars to raise awareness of the work and address any questions.
Proposed structure for BAA Scope of Practice document(s)
General considerations
Across UK healthcare professions, there is no single definition of what constitutes a scope of practice document, partly driven by variation in legislation and registration across the professions[1]. Additionally, there is no single regulatory body for all audiology professionals working in the UK. The regulatory body for audiologists working in the NHS, the Academy of Healthcare Science (incorporating the Registration Council for Clinical Physiologists), remains voluntary. In contrast, audiology professionals working in the private sector (i.e. hearing aid dispensers and clinical scientists) are required to register with the Health & Care Professions Council.
This renders the task of redeveloping the BAA Scope of Practice (ScoP) Document particularly challenging. Equally, it underlines the requirement for a unified scope of practice document for all audiology professionals across sectors.
To ensure the BAA ScoP effectively meets this requirement, the document will be designed and assessed using the regulatory principles defined by Leslie et al., (2021). Nevertheless, the BAA is not a regulatory body, so the ScoP will be shared with a stakeholder group that includes other audiology professional and regulatory bodies, to ensure that it is both fit for purpose and effectively utilised.
Table 1 Taken from the Leslie et al., (2021) analysis of leading practices to regulating scopes of practice across the US, Canada, UK and Australia
| Regulatory principle | Description related to scopes of practice | Leading practices |
| Definition | Clear definitions of professional scope that advance regulators’ mandate of protecting public safety | Uniform legislation and regulatory authorities operating nationally provide consistent and clear practice standards and regulatory frameworks (Australia) |
| Flexibility | Regulation sufficiently flexible and responsive to allow for timely innovation and optimisation in scopes of practice | Umbrella frameworks that offer regulatory flexibility and loosen the restrictiveness of scopes of practice (many Canadian jurisdictions) |
| Accountability | Scope of practice regulation is transparent and contributes to high-quality and safe patient care | Transparent and publicly accountable risk-based processes with separate oversight body (UK) |
| Efficiency | Optimising coherence, coordination, and communication while maintaining focus on public safety | Licensure compacts allowing licensure recognition and sharing of regulatory data across jurisdictions (US) |
| Collaboration | Legitimate stakeholder perspectives included in scope of practice consultations and definitions | Increased public engagement in regulatory processes such as community reference group (Australia) |
Plan for ScoP structure & development
At the initial BAA ScoP working group meeting[2], the purpose of the ScoP was discussed in the context of other UK professional scopes of practice, and the following aims for this work were identified:
- Defining extent and limits of practice for an audiologist.
- Identifying minimum levels of clinical capabilities according to career level and/or context.
- Establishing the principles of good audiological practice, which all audiologists must adhere to, regardless of career framework level or sector, to ensure patient safety.
The broad consensus was for division of this work into 3 sections or 2-3 separate documents.
| Section | Aim(s) | Potential challenges/limitations |
| (A) Scope of Practice
|
Defining the clinical and professional capabilities of an audiologist.
E.g., RCLST guidance.
|
Are we duplicating content across (A) and (C)?
|
| (B) Career Framework | Career levels linked to capabilities.
E.g., GDC ScoP (although they just released a new framework document) and the BAA ScoP (2014).
|
How will this document work across sectors e.g., for audiologists in the private sector, schools or industry?
How will it accommodate changes in practice?
|
| (C) Good Audiological Practice Guide | Setting out expectations of audiologists in practice, including
i. knowledge, skills & performance ii. safety and quality iii. communication partnership and teamwork iv. maintaining trust
E.g., GMC guide.
|
We can set out expectations for good practice, but we cannot mandate them as we are not a regulatory body. |
Next steps
- Allocating responsibility of each section/document development to one lead writer.
- Setting targets for first drafts of each section to be ready for peer review by other group members.
- Setting up stakeholder group for review.
[1] Leslie, K., Moore, J., Robertson, C. et al. Regulating health professional scopes of practice: comparing institutional arrangements and approaches in the US, Canada, Australia and the UK. Hum Resour Health 19, 15 (2021). https://doi.org/10.1186/s12960-020-00550-3
[2] On 5 October 2022
